
Chronic microangiopathy refers to damage occurring in the body’s smallest blood vessels. This widespread condition significantly impacts various organs and a person’s overall health. Understanding this often-overlooked condition is very important. It presents a serious risk to well-being. This blog aims to shed light on microangiopathy, helping readers grasp its complexities and potential for relief.
Key Takeaways
Chronic microangiopathy damages small blood vessels. This condition affects many body parts and overall health.
High blood sugar, high blood pressure, and certain genes can cause chronic microangiopathy. Lifestyle choices like smoking also play a role.
This condition can harm organs like the kidneys, eyes, and brain. It can lead to problems like memory loss or kidney failure.
Doctors use MRI scans and blood tests to find chronic microangiopathy. Early diagnosis helps manage the condition better.
Managing chronic microangiopathy involves controlling blood sugar and blood pressure. Healthy eating, exercise, and medicines also help improve health.
What is Chronic Microangiopathy?

Understanding Small Vessel Damage
Chronic microangiopathy describes a condition where damage occurs to the body’s smallest blood vessels. These include capillaries, arterioles, and venules. This damage impairs normal blood flow. It also leads to tissue damage throughout the body. This microvascular dysfunction affects many organ systems. It compromises their ability to receive oxygen and nutrients. The resulting ischemic conditions can have serious health consequences.
Several cellular mechanisms contribute to this vascular damage in chronic microangiopathy:
Amyloid-beta (Aβ) Deposition: In conditions like cerebral amyloid angiopathy (CAA), Aβ protein builds up in the walls of small arteries. This leads to vascular dysfunction and injury. It causes fibrinoid necrosis, loss of smooth muscle cells, and wall thickening.
Collagen Overexpression: An excess of fibrillar collagen type-III and strong immunoreactivity of collagen type-IV in vascular smooth muscle cells link to non-amyloid microangiopathy. This contributes to the structural changes in the small blood vessels.
Damage to these microvascular structures also involves other critical processes:
Smooth Muscle Cell Degeneration and Loss: This is a key change in microangiopathy. Plasma components or lipids often infiltrate the vascular wall. Macrophages produce reactive oxygen species. They induce smooth muscle cell hypertrophy. They also secrete matrix metalloproteinases (MMPs). Fibrinogen infiltration can inhibit peroxisome proliferators-activated receptors in smooth muscle cells. This increases C-reactive protein and MMP-9 expression. Smooth muscle cells can also change from a contractile to a synthetic state. This further impairs vascular function.
Endothelial Dysfunction: The endothelium lines the inside of blood vessels. Its dysfunction involves a reduced release of nitric oxide (NO). NO is vital for regulating cerebral blood flow and mediating vascular dilation. Elevated levels of endothelin-1 (ET1), a vasoconstrictor, also contribute. This disrupts the balance between NO and ET1. This imbalance leads to poor microvascular regulation and increased ischemic risk. The overall effect is widespread ischemic injury due to compromised microvascular networks.
Causes of Chronic Microangiopathy
Chronic microangiopathy develops from a combination of systemic conditions, genetic predispositions, and lifestyle choices. These various factors contribute to the damage of the body’s delicate vascular network. Understanding these causes helps in prevention and management.
Systemic Conditions
Several systemic diseases are primary drivers of chronic microangiopathy. These conditions create an environment harmful to the small blood vessels.
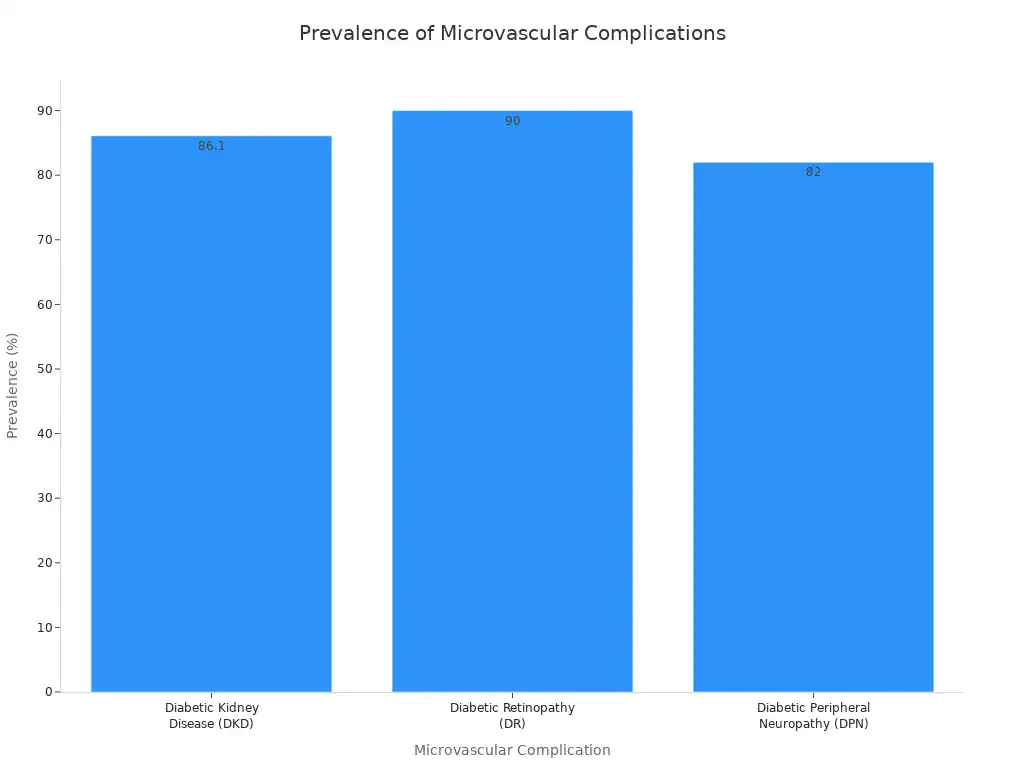
Diabetes mellitus, characterized by high blood sugar (hyperglycemia), significantly contributes to microvascular damage. High glucose levels directly harm the endothelial cells lining the small blood vessels. This leads to inflammation and oxidative stress. Over time, these changes impair blood flow and tissue function. Individuals with type 2 diabetes often experience various microvascular complications.
Microvascular Complication | Pooled Prevalence of Depression |
|---|---|
Neuropathy | 43% |
Retinopathy | 40% |
Nephropathy | 18% |
A systematic review shows a high prevalence of cognitive impairment and depression among individuals with type 2 diabetes. These individuals also experience microvascular complications. These complications include retinopathy, neuropathy, and nephropathy. The impact of diabetes on the vascular system is widespread.
High blood pressure, or hypertension, also places immense stress on the vascular system. This constant pressure damages the walls of the small blood vessels. It makes them stiff and narrow. This reduces their ability to deliver blood effectively. Hypertension is a major risk factor for various forms of microangiopathy.
Autoimmune diseases, such as lupus and scleroderma, also cause chronic microangiopathy. These conditions involve the immune system mistakenly attacking the body’s own tissues. This attack often includes the vascular structures. This leads to inflammation and damage in the microvascular beds. These systemic conditions are significant risk factors for developing chronic microangiopathy. They represent key vascular risk factors.
Genetic Factors
Genetics play a role in an individual’s susceptibility to chronic microangiopathy. Certain genetic variations can increase the risk of developing the condition. These genetic factors influence how the body handles blood pressure, inflammation, and vascular repair.
Specific genetic markers are linked to an increased risk of chronic microangiopathy:
The AGT gene, specifically the M235T codon, is associated with the progression of brain injury in chronic microangiopathy.
The -20A > C polymorphism in the AGT promoter region is associated with leukoaraiosis in hypertensive patients.
The B-haplotype of the AGT gene (–6:A, –20:C, –153:G, –218:G) is a hypertension-independent risk factor for brain changes. B/B homozygosity increases the risk eight-fold or more.
The PLAT (TPA) gene polymorphism rs2020918 (-7351C/T) has contradictory data regarding its association with lacunar infarcts.
The AA genotype of polymorphism rs1800790 (455G/A) of the FGB gene is associated with an increased risk of multiple lacunar infarcts.
The IL6 gene polymorphism rs1800796 (572G > C) is associated with the development of hypertension in the Asian population.
The TNFα gene polymorphism rs1800629 (308G > A) is associated with the development of hypertension in the Asian population. It also affects the course of hypertension in the Russian Central Chernozem Region.
The MMP9 gene polymorphism rs3918242 (–1562C > T) is associated with a risk of hypertension.
The EDN1 gene polymorphism rs5370G>T (codon K198N) is associated with the development of hypertension.
Combinations of MTHFR C677T with the ACE gene’s D/D genotype worsen chronic microangiopathy (white matter hyperintensities, lacunar infarcts).
Combinations of APOE gene genotypes (2/2, 2/3, 4/4, or 4/3) with MTHFR C677T or ACE D/D genotype can act as an independent genetic risk factor for leukoaraiosis.
The MTHFR C677T and A1298C polymorphisms are linked to microangiopathy in type 2 diabetes mellitus.
These genetic factors highlight the complex interplay between inherited traits and vascular health. They represent important risk factors.
Lifestyle and Environmental Influences
Lifestyle choices and environmental exposures significantly impact the health of small blood vessels. These factors can exacerbate existing conditions or independently contribute to vascular damage.
Smoking is a major risk factor. It directly harms the endothelium and promotes inflammation. This leads to narrowing and hardening of the small blood vessels. High cholesterol levels also contribute to vascular damage. Excess cholesterol can build up in the vessel walls.
This forms plaques that impede blood flow and trigger inflammatory responses. Chronic inflammation, regardless of its origin, consistently damages the vascular system. It creates an environment where microvascular injury is more likely. These are all important vascular risk factors.
Environmental factors also play a role. Long-term exposure to air pollution contributes to the development of chronic microangiopathy.
Studies indicate that higher particulate matter exposure in diabetic patients can lead to an increased risk of diabetic retinopathy (DR). This is a microangiopathic change in the eyes.
A retrospective cohort study found that atmospheric air pollution, including PM2.5, PM10, NO2, SO2, and O3, was linked to a higher risk of central retinal artery obstruction (CRAO). This was particularly true in individuals with diabetes or hypertension and those over 65.
Long-term exposure to carbon monoxide (CO) and PM2.5 has been shown to increase albuminuria in type 2 diabetes patients. This indicates renal dysfunction and microvascular damage in the kidneys.
These environmental factors add to the overall burden on the vascular system. They increase the risk of ischemic damage. All these factors, both genetic and environmental, contribute to the complex picture of chronic microangiopathy. They are critical risk factors for this condition.
Risks and Complications
Chronic microangiopathy poses significant risks to various organ systems. It leads to a range of complications. These complications arise from impaired blood flow and tissue damage. Understanding these risks helps in early detection and management.
Organ-Specific Impacts
Chronic microangiopathy affects many organs. The kidneys often suffer damage. This leads to nephropathy and can progress to renal failure. Pathological changes in kidney tissue include double contours of the basement membrane and mesangiolysis.
Endothelial swelling and glomerular paralysis also occur. Thrombi in arteries and fibrin thrombi in glomerular capillary lumina are common. Erythrocyte fragments appear within arterial vessel walls. Mucoid thickening and obliteration of small artery lumens are present.
Myxoid intimal thickening of small arteries and fibrinoid necrosis of arterial walls also contribute to kidney damage. Reduplication of the glomerular basement membrane and fibrillary mesangium are other signs.
Immunofluorescent studies show granular capillary loops and mesangial staining for fibrinogen, IgM, IgG, IgA, and C3. Electron microscopy reveals segmental fusion of foot processes, glomerular endothelial swelling (endotheliosis), lipid vacuoles, and double contour of glomerular basement membranes with subendothelial expansion. Mesangial and paramesangial electron dense deposits, likely fibrin, are also visible.
The eyes are also vulnerable. Retinopathy, a common complication, can lead to blindness. The heart can develop cardiomyopathy and heart failure due to microvascular damage. These are all serious consequences of microvascular ischemic disease.
Cerebral Microangiopathy and Brain Health
Cerebral microangiopathy, also known as cerebral small vessel disease, significantly impacts brain health. It plays a role in lacunar infarcts, which are small ischemic strokes.
This condition also contributes to dementia and cognitive impairment. Microvascular ischemic disease in the brain can lead to progressive cognitive decline. Post-mortem analyses of individuals with dementia often show cerebral small vessel disease lesions.
These include microinfarcts, microbleeds, and arteriolosclerosis. These are common pathologies that increase the odds of dementia. Dysfunction of the blood-brain barrier and reduced cerebral vasoreactivity are also involved. Structural abnormalities can decrease blood-brain barrier integrity.
This leads to extravasation of blood proteins that damage brain tissue. Impaired activity-related changes in cerebral blood flow cause chronic and intermittent hypoperfusion. This leads to white matter hyperintensities.
Cerebral microangiopathy contributes to dementia through various mechanisms. These include thickening of vessel walls, blood-brain barrier disturbance, and reduced perfusion rates.
Disturbed vasomotor reactivity, edema, ischemia, demyelination, axon loss, and gliosis also occur. These processes lead to focal and diffuse lesions in the subcortical, deep, and periventricular white matter. Lacunes also appear in the central grey matter.
Cortical microinfarcts, a feature of cerebral microangiopathy, predict cognitive decline. A total cerebral small vessel disease score, integrating various neuroimaging markers, associates with cognitive decline in executive function. A more granular measure of microangiopathy severity, like the Fazekas scale, better indicates its impact on cognitive function. This suggests that risk factors for microvascular ischemic disease are crucial to monitor.
Other Systemic Risks
Beyond major organs, microvascular ischemic disease presents other systemic risk factors. Peripheral neuropathy, affecting nerves in the extremities, causes pain, numbness, and weakness. Impaired wound healing is another significant risk.
Poor blood supply to tissues delays recovery and increases infection susceptibility. Thrombotic microangiopathy (TMA) is a severe complication. It involves widespread formation of tiny blood clots in small blood vessels. This can lead to organ damage and failure. These symptoms of microvascular ischemic disease highlight its widespread impact.
Chronic microangiopathy damages small blood vessels. Its diverse causes and significant risks affect various organs, including the brain. It can lead to cerebral complications.

